Vapor Pressure#
Vapor Pressure = {model_name} <species> {float_list} [varies]
Description / Usage#
This required card is used to specify the model for the vapor pressure for each species; it has two main classes of use. The first class regards multiphase flow in porous media, which is activated when the media type is set to POROUS_UNSATURATED or TWO_PHASE (cf. the Media Type card). The second class of use of this data card is for specification of vapor pressure at the external boundary of a liquid domain, for which the bounding gas phase is modeled with a lumped parameter approach, or at an internal interface between a liquid and a gas. No curvature effects are included here. Eventually the models in this class will be supported in the porous-media cases. Definitions of the input parameters are as follows:
{model_name} |
Name of the model for the vapor pressure, based on the class of use. For the first class of multiphase flows in porous media, {model_name} can be one of the following:
For the second class regarding specification of vapor pressure at the external boundary of a liquid domain or the interface between a gas and a liquid, {model_name} can be one of the following:
|
<species> |
An integer designating the species equation. Typically this value is zero if the problem is one of a single solvent in a partially saturated medium. |
{float_list} |
One or more floating point numbers (<float1> through <floatn>) whose values are determined by the selection for {model_name}. |
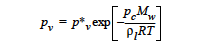
Vapor pressure model choices and their parameters are discussed below.
Models in the first class…
KELVIN <species> <float_list> |
The <float_list> for the KELVIN option specifies input values for seven parameters:
The KELVIN option is used to include the effect of vapor-pressure lowering that results in equilibrium over high curvature menisci, i.e., small pores. The equation form of this is |
FLAT <species> <float_list> |
The FLAT option requires the same seven parameters as the KELVIN model but leaves off the exponential function, i.e., the vapor pressure is independent of the level of capillary pressure. The constants are still needed so that the gas-phase concentration can be calculated with the ideal gas law. See the KELVIN option above for definition of the <float_list> values. |
IDEAL_GAS <species> <float_list> |
The <float_list> for this model has three values, where:
|
Models in the second class..
CONSTANT <species> <float1> |
This model is used for a constant species source such as a homogeneous reaction term. The <float_list> has a single value:
|
ANTOINE <species> <float_list> |
The ANTOINE model for vapor pressure is used in conjunction with the VL_EQUIL boundary condition. If specified, a temperature-dependent vapor pressure for species i is calculated. The model requires six values in the <float_list>, where:
|
RIEDEL <species> <float_list> |
The RIEDEL model for vapor pressure is used in conjunction with the VL_EQUIL boundary condition card. If specified, a temperature-dependent vapor pressure for species i is calculated. The model requires eight values in the <float_list>, where:
|
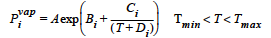
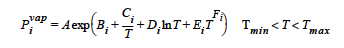
Examples#
An example use of the Antoine model for vapor pressure follows:
Vapor Pressure = ANTOINE 0 1 9.380340229 3096.516433 -53.668 0.1 1000
Technical Discussion#
No Discussion.
References#
No References.
