Chemical Potential#
Chemical Potential = {IDEAL_SOLUTION | STOICHIOMETRIC_PHASE}
Description / Usage#
This card is used to specify the formulation of the chemical potential for the phase. It is currently unconnected to Goma’s functionality. Two values are permissible:
IDEAL_SOLUTION |
Ideal solution thermodynamics |
STOICHIOMETRIC_PHASE |
Phase consists of fixed set of molecular composition |
Examples#
Following is a sample card:
Chemical Potential = IDEAL_SOLUTION
Technical Discussion#
The chemical potential of species k in an ideal solution is given by the expression, [Denbigh, p. 249],
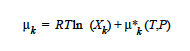
where μk*(T, P) is defined as the chemical potential of species k in its pure state (or a hypothetical pure state if a real pure state doesn’t exist) at temperature T and pressure P. μk*(T, P) is related to the standard state of species k in the phase, μk, o(T), which is independent of pressure, through specification of the pressure dependence of the pure species k. Xk is the mole fraction of species k in the phase.
The chemical potential of species k (actually there is only one species!) in a stoichiometric phase is equal to
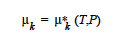
References#
Denbigh, K., The Principles of Chemical Equilibrium, 4th Ed., Cambridge University Press, 1981
